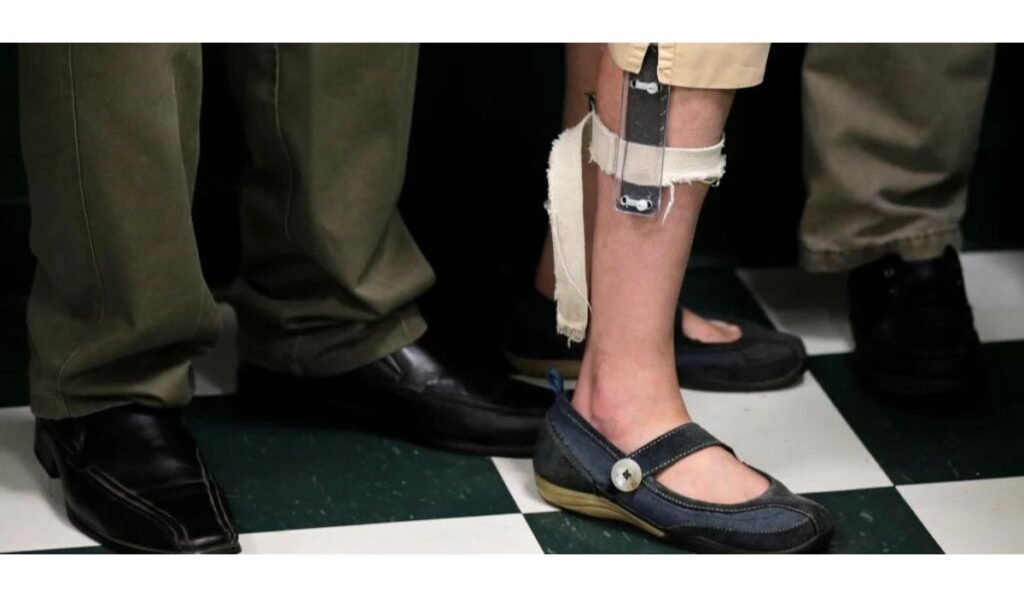
Amid mounting safety concerns, the FDA has decided to renew its proposed ban on electrical stimulation devices. These devices, often used in the treatment of various conditions, have sparked controversy due to their potential adverse effects on users. Consequently, the FDA is taking proactive steps to mitigate these risks and safeguard public health by moving forward with the proposed ban.
In response to compelling scientific evidence highlighting the risks associated with electrical stimulation devices, the FDA is moving forward with its proposed ban on such products. Extensive research has underscored the potential for skin irritation, burns, and psychological distress resulting from prolonged exposure to electrical stimulation. Consequently, the FDA is taking proactive measures to protect individuals, especially those vulnerable to adverse effects, by enacting the ban on these devices. The FDA aims to prevent further harm and promote the well-being of individuals who may be vulnerable to the adverse effects of these devices.
The FDA’s proposed ban underscores the importance of thorough evaluation and regulation of medical devices to ensure their safety and efficacy. While electrical stimulation devices may offer therapeutic benefits for some individuals, the potential risks outweigh the benefits in many cases. By implementing this ban, the FDA aims to protect consumers from unnecessary harm and promote the use of safer alternative treatments.
read more
image source